Current Research
Propagation of noise from mRNA to protein
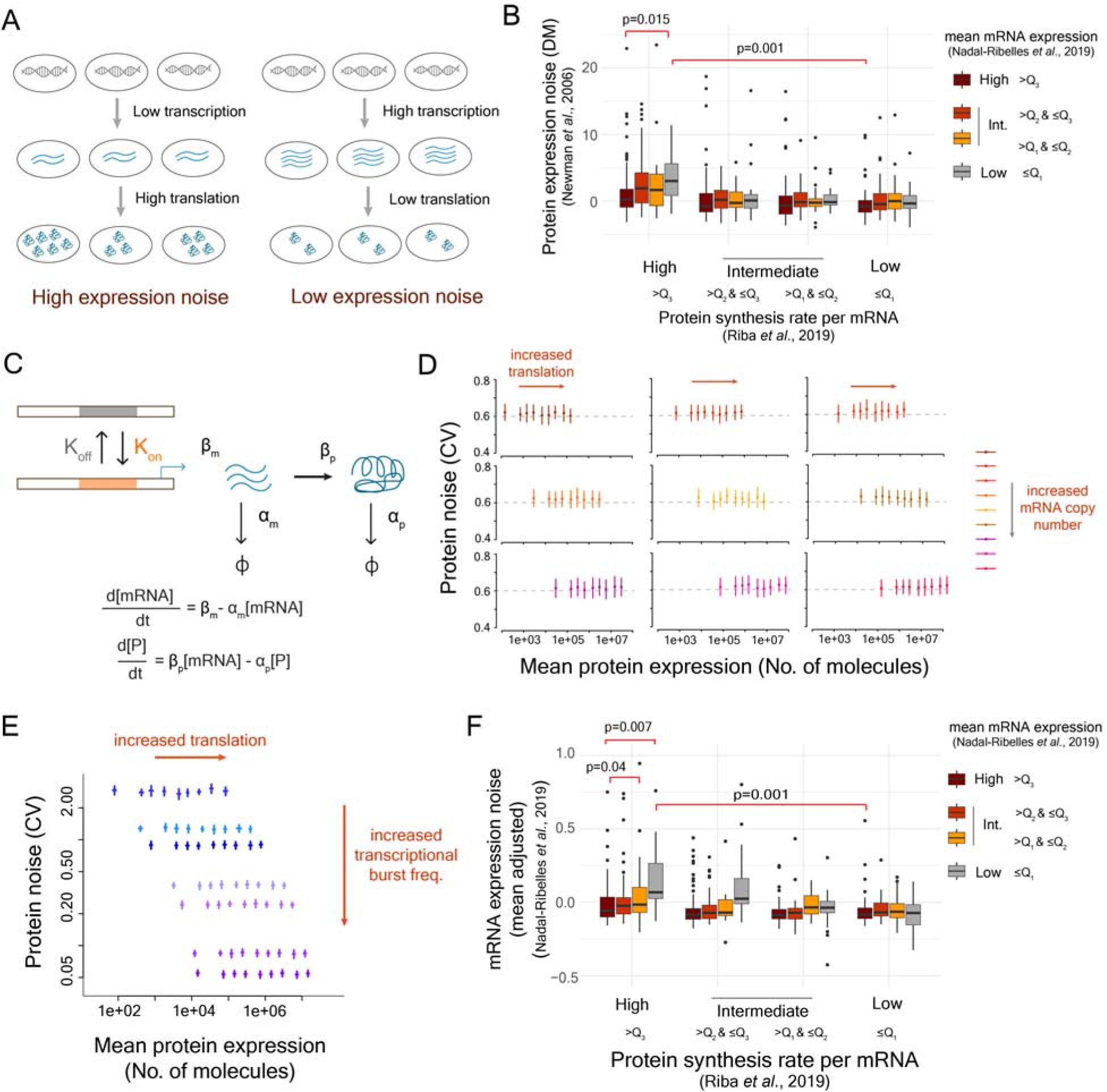
Stochastic variation in protein expression generates phenotypic heterogeneity in a cell population and has an important role in antibiotic persistence, mutation penetrance, tumor growth and therapy resistance. Studies investigating molecular origins of noise have predominantly focused on the transcription process. However, the noise generated in the transcription process is further modulated by translation. This influences the expression noise at the protein level which eventually determines the extent of phenotypic heterogeneity in a cell population. Studies across different organisms have revealed a positive association between translational efficiency and protein noise. However, the molecular basis of this association has remained unknown. In this work, through stochastic modeling of translation in single mRNA molecules and empirical measurements of protein noise, we show that ribosome demand associated with high translational efficiency in a gene drives the correlation between translational efficiency and protein noise. We also show that this correlation is present only in genes with bursty transcription. Thus, our work reveals the molecular basis of how coding sequence of genes, along with their promoters, can regulate noise. These findings have important implications for investigating protein noise and phenotypic heterogeneity across biological systems.
Cryptic vs de-novo mutation: Role in adaptation
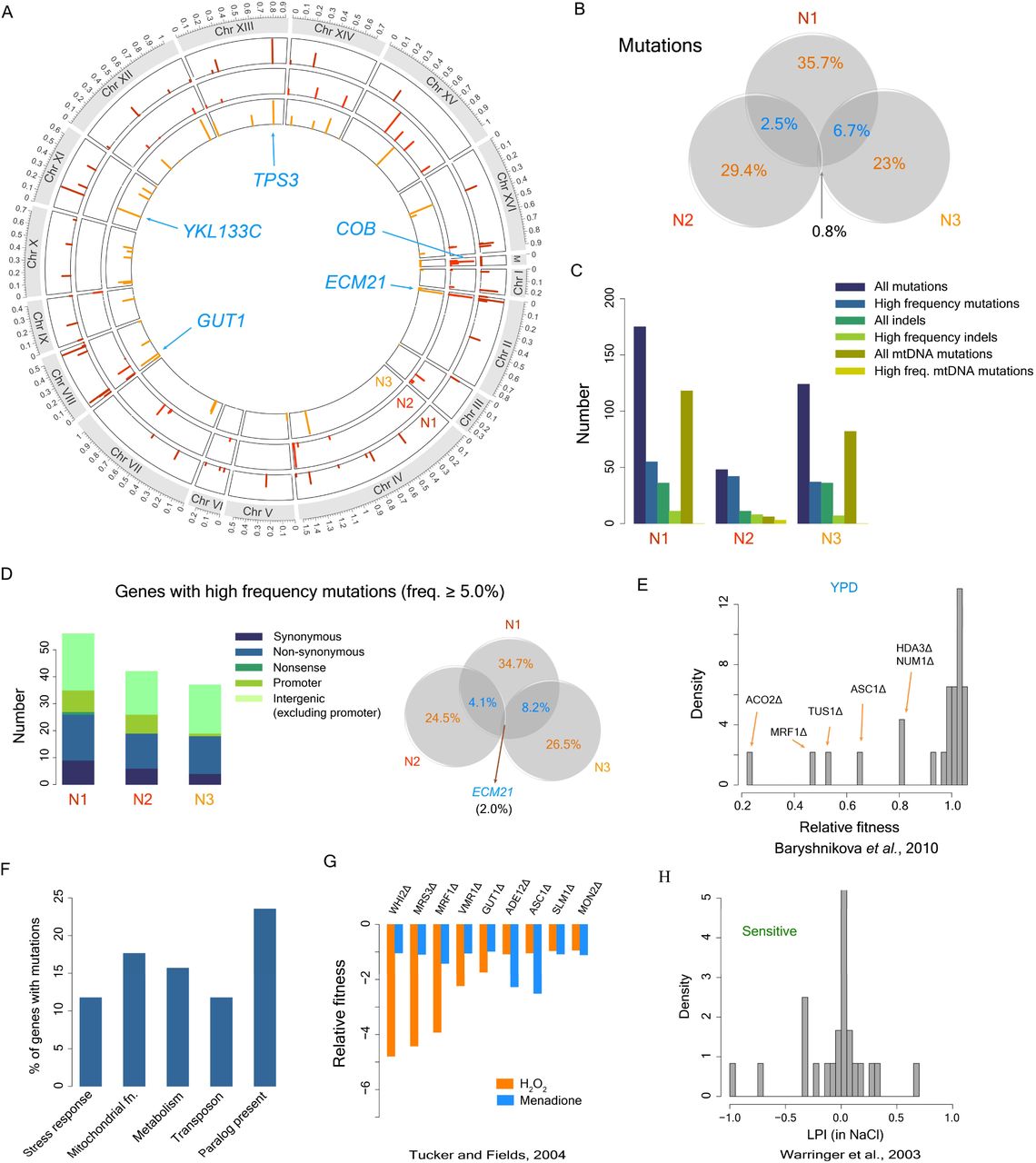
Many organisms live in predictable environments with periodic variation in growth condition which can allow populations to accumulate cryptic genetic variations. Cryptic variations can facilitate adaptation to new environments, as observed in evolution experiments with a ribozyme and a protein. Whether the same holds for cell populations remains unclear. Alternatively, living in a near-constant condition can lead to loss of nonessential cellular functions, which could be maladaptive in new environments. Through laboratory evolution experiments in yeast, we show that populations grown in a predictable nutrient-rich environment for 1000 generations start to lose their ability to respond and adapt to new stressful environments. Growth of yeast populations in the nutrient-rich environment was associated with modest fitness increase in this environment, metabolic remodeling, and increased lipid accumulation. In novel stressful environments, however, these populations generally had reduced fitness, except in salt-stress where lipid accumulation seemed to provide osmotic protection. We further found that adaptation to stressors was primarily driven by de novo mutations, with very little contribution from the mutations accumulated prior to the exposure to stressors. Thus, our work suggests that in the absence of occurrence of new environments, natural populations might not accumulate cryptic variations that could be beneficial for adaptation to these environments. In addition, presence of selection in predictable condition in natural populations may purge away some of the cryptic variations. Taken together, these findings raise questions about persistence of cryptic variations in natural populations and their importance in evolutionary adaptation.
Transcriptional noise: Contribution towards cancer progression
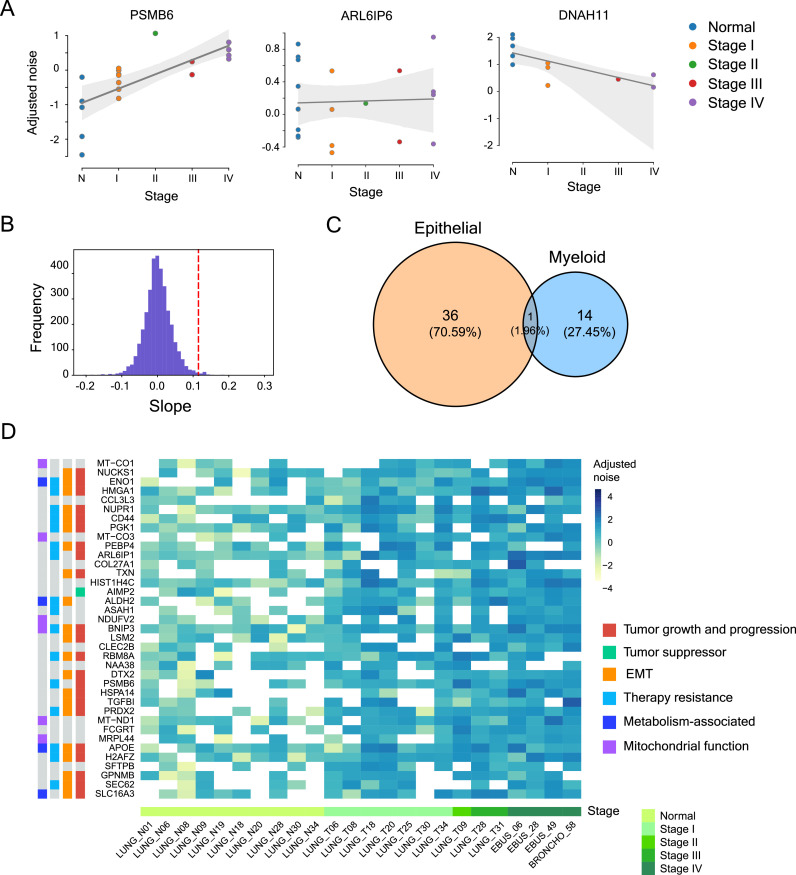
Fluctuations in the number of regulatory molecules and differences in timings of molecular events can generate variation in gene expression among genetically identical cells in the same environmental condition. This variation, termed as expression noise, can create differences in metabolic state and cellular functions, leading to phenotypic heterogeneity. Expression noise and phenotypic heterogeneity have been recognized as important contributors to intra-tumor heterogeneity, and have been associated with cancer growth, progression, and therapy resistance. However, how expression noise changes with cancer progression in actual cancer patients has remained poorly explored. Such an analysis, through identification of genes with increasing expression noise, can provide valuable insights into generation of intra-tumor heterogeneity, and could have important implications for understanding immune-suppression, drug tolerance and therapy resistance. In this work, we performed a genome-wide identification of changes in gene expression noise with cancer progression using single-cell RNA-seq data of lung adenocarcinoma patients at different stages of cancer. We identified 37 genes in epithelial cells that showed an increasing noise trend with cancer progression, many of which were also associated with cancer growth, EMT and therapy resistance. We found that expression of several of these genes was positively associated with expression of mitochondrial genes, suggesting an important role of mitochondria in generation of heterogeneity. In addition, we uncovered substantial differences in sample-specific noise profiles which could have implications for personalized prognosis and treatment.